A New Approach: Targeted Mitochondrial Action
Our groundbreaking drug candidates are designed to accumulate in mitochondria and to directly address mitochondrial function and increase energy expenditure. This allows low doses of the drug to regulate key biological processes involved in many serious diseases, such as rare mitochondrial disorders and metabolic disorders, offering new ways to deliver significant therapeutic benefits.
Metabolic diseases
- Weight loss of 27% in a large non-rodent HFD animal model after two months of treatment.
- Muscle mass remained unaffected.
- Significant synergetic weight loss efficacy in a combination with GLP-1 agonist treatment vs both monotherapies.
- Weight loss maintenance after discontinuation of optimal doses of GLP-1 agonists (for both semaglutide and tirzepatide).

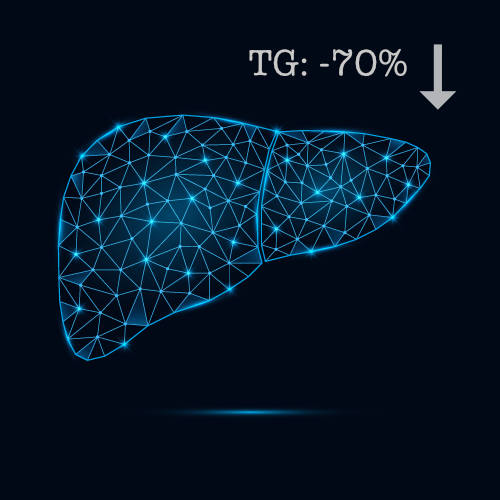
In preclinical MASH models, our treatment led to significant improvements in several key biomarkers associated with liver health and metabolic function.
- Liver triglyceride levels were significantly reduced – by 70%.
- Significant decreases in steatosis, ballooning, and fibrosis.
- Significant reduction in oxidative stress biomarkers, further supporting the molecule’s role in mitigating liver damage.
Neurodegenerative Disorders
Mitotech is pioneering a "first-in-class" small molecule designed to specifically target and inhibit ferroptosis at its origin—within the mitochondria. By blocking ferroptosis, particularly in neurons within the central nervous system ( CNS), our lead candidate holds promise for halting or slowing the progression of neurodegenerative diseases.
Our lead candidate demonstrated robust efficacy in models of Friedreich’s Ataxia (FA) and Multiple Sclerosis (MS).
- Protection of FA patient fibroblasts from ferroptosis-mediated cell death in vitro using low doses of SkQ1 alone and enhanced protection in combination with omaveloxolone.
- Significantly reduced disease scores and histology inflammation scores, as well as reduced plasma levels of neurofilament light chain (Nfl), in mouse EAE model of MS.
